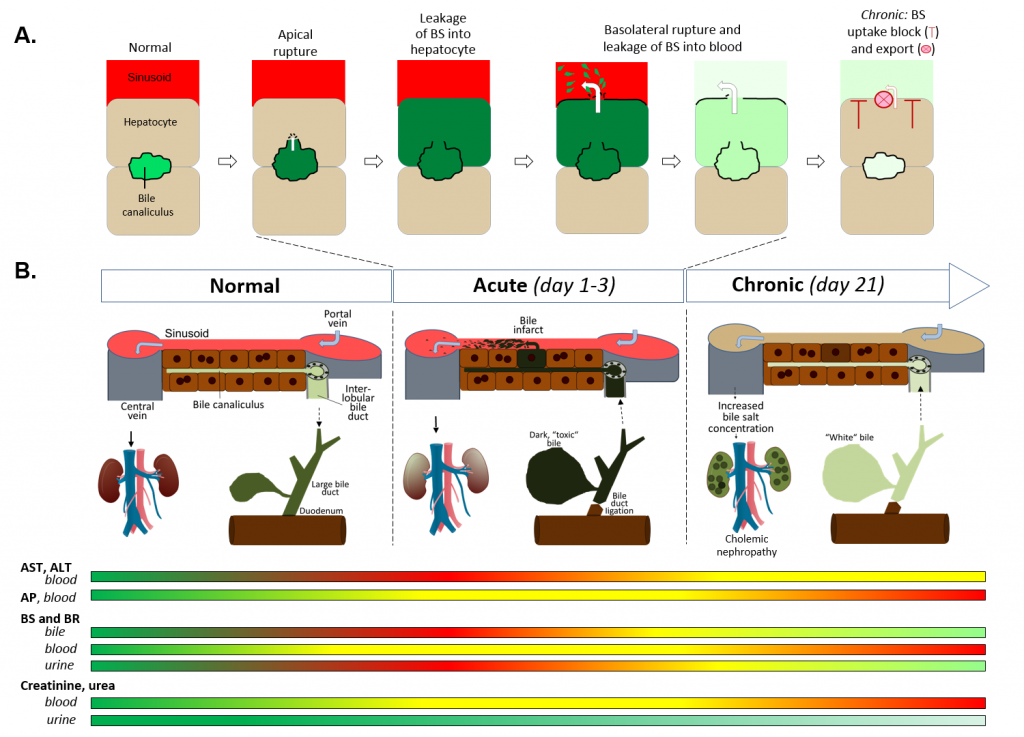
Bile duct ligation (BDL) is a frequently applied experimental procedure with the aim to mimic human cholestatic disease. One of the early consequences of BDL are so-called ‘bile infarcts’ in rodents that correspond to Charcot-Gombault necrosis in human cholestasis. The mechanisms causing bile infarcts and their pathophysiological relevance remain unclear. Therefore, intravital two-photon-based imaging of common bile duct-ligated mice was performed with fluorescent BS and non-BS organic anion analogues. Key findings were followed-up by MALDI imaging, clinical chemistry, immunostaining and gene expression analyses. In the acute phase after BDL, BS concentrations in bile are increased and single cell bile micro-infarcts occur caused by apical hepatocyte membrane rupture after loss of mitochondrial membrane potential. This leads to cell death by entry of bile, followed by a ‘domino effect´ of further death events of neighboring hepatocytes. Bile infarcts provide a trans-epithelial shunt between bile canaliculi and blood by which bile constituents leak into blood. In the chronic phase, uptake of BS tracers at the sinusoidal hepatocyte membrane was reduced, which contributes to elevated concentrations of BS in blood and decreased concentrations in the biliary tract. Conclusion: bile micro-infarcts occur in the acute phase after BDL in a limited number of dispersed hepatocytes followed by larger infarcts involving neighboring hepatocytes. These infarcts allow leakage of BS and other solutes from the biliary tract into blood, thereby reducing their concentrations in the canalicular network. This protects the liver from BS overloading. In the chronic phase after BDL reduced sinusoidal BS uptake appears to be a dominant protective factor. The kidney contributes to the elimination of BS until cholemic nephropathy sets in.